Seeding experiments allow to improve the likelihood of successful protein crystallization and to optimize the growth conditions.
In order to crystallize a biological macromolecule, its concentration is slowly increased until a point of supersaturation is reached. Using the phase diagram,
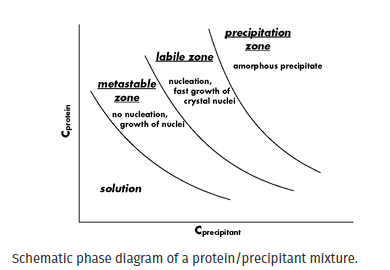
supersaturation can be displayed in three successive zones, i.e. metastable supersaturation, labile supersaturation and precipitation (Fig. 1). In the metastable zone, no spontaneous nucleation can occur but crystals added to this zone can grow. In the labile zone, spontaneous nucleation takes place and fast growth of the nuclei is observed. In the precipitation zone, the biological macromolecule is many times supersaturated leading to a formation of amorphous precipitate.
Nucleation occurs at a higher level of supersaturation than crystal growth. By placing seeds into a solution supersaturated in the metastable zone, the growth conditions can be optimized and large single crystals can be obtained.
The number of crystals grown can be influenced by the concentration of the seed stock which is added to the protein drop. Seeding with a very concentrated seed stock can result in showering of microcrystals. If the seed stock is too dilute, no nuclei will be transferred to the protein drop. The ideal concentration of the seeding solution can be determined experimentally by performing serial dilutions from a concentrated seed stock.
Instructions for preparation of a seed stock
- Preparation of a stabilizing solution
A stabilizing solution should maintain the stability of a crystal, i.e. the crystal should not dissolve nor grow any further. Ideally, it should have the same composition as the solution of the drop from which the crystal was removed. This can be experimentally achieved by mixing the original sample solution with the drop reservoir. - Pipet 50 μl of stabilizing solution to the microcentrifuge tube containing the glass bead.
- Harvest a crystal from the drop using a MicroMount or MicroMesh inserted into a 0.7 mm mechanical pencil for better control. Remove excess liquid with a paper wick to minimize the carryover of liquid.
- Place the crystal in the microcentrifuge tube containing the glass bead and the stabilizing solution. Close the tube tightly.
- Vortex at medium speed for ca. 2 min. The glass bead should randomly bounce in the tube to ensure effective crushing.
- Add 450 μl stabilizing solution and mix thoroughly.
- Serial dilutions of the seed stock can be prepared by 1:10 dilutions of seed stock with stabilizing solution. The seed stocks may be successively diluted up to 10-5 times of its original concentration.
Please note: If your stabilization solution contains detergents or other additives which may foam vortexing is not recommended. Alternatively, the sample is treated exactly as described above, but instead of vortexing it is placed in an ultrasonic cleaner for two 1 min intervals.
Use a new microcentrifuge tube and a clean glass bead each time you prepare a new seed stock to avoid contamination and carryover from past experiments.
Performing the crystallization experiment
Prepare the crystallization drop by mixing protein sample and seed stock. Do not add reservoir solution since this may dissolve the seeds. Place the drops over a reservoir solution which has the same composition as the reservoir that was used to grow the initial seed crystal.
Ideally, the seeds should be transferred to a protein solution in the metastable zone, i.e. the solution should be slightly supersaturated.
Transferring seeds to an under saturated solution would result in the dissolution of the nuclei and no crystal growth would be observed. Transferring seeds to a highly supersaturated solution can yield showers of microcrystals.