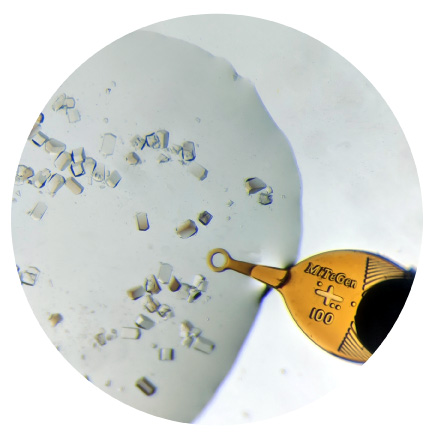
MicroMounts™ are the world’s highest performance tools for retrieving and mounting protein crystals, virus crystals, and small molecule/inorganic crystals. They can be also used for handling small fragile samples of all sorts, including biological, archaeological and mineralogical samples. MicroMounts™ are compatible with all standard X-ray hardware, and can be inserted in 0.7 mm mechanical pencils or micromanipulators for easy handling.
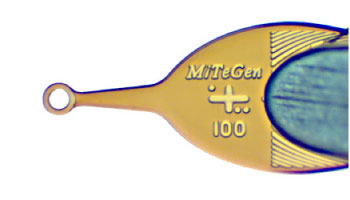
Micromounts™ consist of thin polymer tips attached to beveled non-magnetic 0.025″ (0.64 mm) diameter stainless steel rods. Their patented design provides an excellent combination of X-ray and optical transparency, mechanical rigidity and flexibility, and precision dimensions.
The polymer is unoriented, so the background scatter does not show the sharp peaks of competing mounts, and has the lowest possible birefringence.
Curving the tip by wrapping its base around the rod makes it rigid, and provides a scoop-like action in retrieving samples. The tip can also flex and bend flat against e.g., a well or glass slide, to dislodge and then slip under a sample.
A wicking aperture draws excess liquid away from the sample, reducing background scatter and allowing faster sample cooling for cryocrystallography.
Since 2008, MicroMounts™ (and all of our other products) have been made using the most mechanically robust unoriented polymer available. With proper care – especially during cleaning – each MicroMount™ can be used to harvest and measure dozens of crystals.
Extremely easy to use, novices can be mounting samples in minutes. MicroMounts™ make your crystallography pipeline more efficient by maximizing the odds of successful mounting, cooling and data collection.
MicroMounts™ consist of a thin microfabricated polyimide film attached to a solid non-magnetic 0.025″ (0.64 mm) stainless steel rod.
- Films
-
Polyimide:
Used in Kapton® tape, which is employed for X-ray transparent windows on X-ray beam lines. Has low atomic number (Z) constituents and low density, and produces less background scatter than e.g., nylon. Optically transparent with an orange-gold hue.
Film Curvature:
Obtained by wrapping polyimide film around the steel rod. Provides excellent stiffness even with very thin (7.5 micrometer) films, and a convenient, scoop-like action in retrieving and handling samples.
- Film Pattern
-
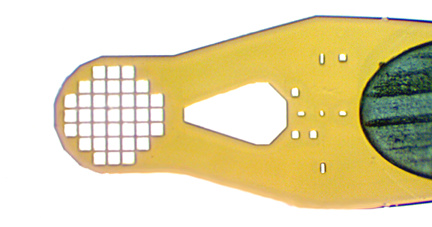
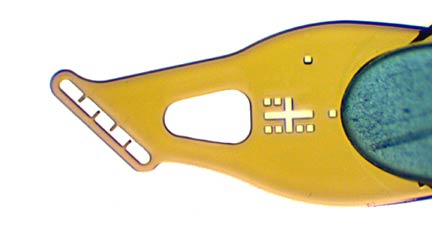
Sample aperture (A) sizes from 10 to 600 micrometers, with minimal polyimide width (B) around the aperture to minimize background scatter in all orientations.
Wicking hole (C) connected via drainage channel (D) to the sample aperture. Hole size is compatible with size 15 or XF paper wicks. “Fountain-pen”-like design allows easier removal of excess liquid – without ever touching the sample with the wick.
Alignment cross (E) located a fixed distance from the center of the sample aperture. Allows easy automated alignment of the sample aperture (A).
Sample aperture size code (F) around the alignment cross. Allows automated recognition of mount design and of aperture and sample size, which determines boundaries of aperture (to be avoided), required alignment tolerance, and area of sample available to be scanned for optimum diffraction. Initial beam size can then be automatically selected. Aperture size (G) in μm can be read directly off the mount.Orientation mark (H) allows the front and back of the mount to be automatically distinguished.
- Rods
- Solid 316 non-magnetic stainless steel rods. Diameter of 0.025″ (0.64 mm) is compatible with all standard goniometer bases / caps, and can be inserted and held in 0.7 mm mechanical pencils. Rods will not be pulled out or trapped by magnets in tools or in automounting hardware. Available in four standard lengths: 11 mm, 19 mm, SPINE, and 25 mm. The 11, 19 and 25 mm rod lengths give rod base-to-sample center distances comparable to those of nylon loops mounted in 10, 18, and 24 mm rods, respectively. Custom rod lengths available on request.
Unlike competing mounts in hollow rods, our solid rods will not suck up samples and liquid.
Beveled at film end. Allows good visibility of the sample for a wider range of rod orientations during sample retrieval than unbeveled rods.
Can be cut using spring-steel-compatible pliers/cutters to any desired length. Can be easily bent to place sample in desired orientation. Fit all standard bases.
The best and easiest way to clean MiTeGen’s tools is to soak them in a bath of a detergent-containing solution. When mounted in MiTeGen base holders, large quantities of mounts can be cleaned at one time.
To remove protein residues, we recommend an enzyme-containing detergent such as Alconox’s Tergazyme or Decon’s Contrex EZ; otherwise, a standard laboratory glassware/plasticware cleaner like Alconox is adequate. We recommend an initial 10 to 15 minute soak, followed by a quick rinse in a bath of water to remove the detergent. The optimal length of the soak needed will be determined by what residual materials are left on the mount or tool (protein, etc.) and how long it has been sinced they were last used. The sooner you clean them after use the easier it will be.
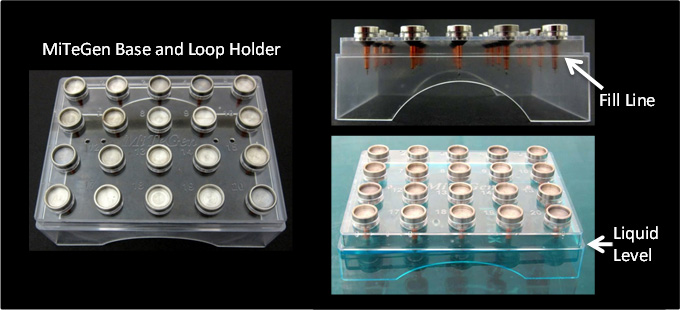
MiTeGen’s Base Holder makes cleaning easy:
|
|
•Load your loop-containing bases into the holder.
•Then place the holder into a plastic or glass tub or dish.
•Mix your detergent-containing solution according to the instructions on the detergent box.
•Then pour the detergent into the tub until the level rises to the Fill Line on the base holder.This will ensure that the loop tips are soaked, while keeping the stainless steel part of the bases out of the solution and preventing corrosion. |
| In the rare case when soaking doesn’t remove deposits, you can use a Medium (M) or Fine (F) paper wick. For easiest handling, cut back from the widest end using a razor blade so that the diameter fits into a 0.7 mm mechanical pencil. Then dip the other end in water, a detergent solution or in isopropanol, and gently stroke the polymer from base to tip. |
|
Handling Do’s and Don’ts:
The microfabricated tips of MiTeGen’s MicroMounts™, MicroLoops™ and other tools are made from the most durable unoriented polymer available, for ultra-low background X-ray scatter. Compared with an oriented fiber like nylon, unoriented polymers can be more easily torn or cut if used improperly. Follow the instructions below to get the maximum life out of our products.
1. Never touch the tips with your hands. Always use our heavy duty tweezers along the pin shaft, not the tip. |
2. Never push the beveled steel pin into the tip against a hard surface.
3. DO: Clean the tips by soaking in detergent while held in a MiTeGen Base Holder.
DO NOT: sonicate for extended times. Sonication for a few minute or so is ok, but sonication for long periods can damage some tips. Using an enzyme-containing detergent, sonication should be unnecessary.

4. DO: Remove stubborn residues using a MiTeGen paper wick
DO NOT: use cotton or foam-tipped swabs. Swabs can catch and tear the polymer.
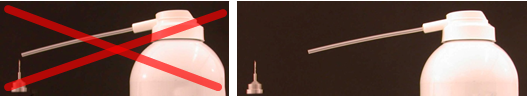
5. To dry the tips after cleaning, let them air dry. You can briefly dip in isopropanol for faster drying. Avoid blow drying. If the nozzle is placed too close to the tip, the resulting turbulent drag force can damage the tip. Keep the nozzle at least 20 cm away from the tip.